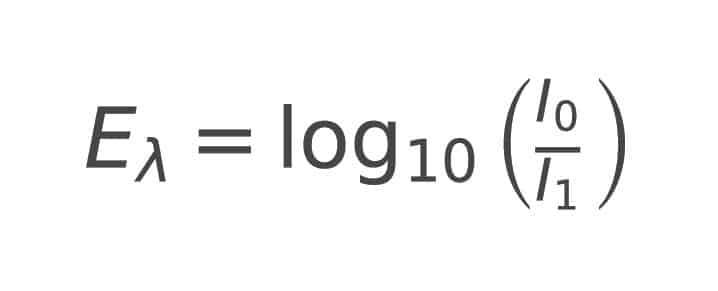A portion of electromagnetic radiation strikes the sample and is converted into other energy states within the sample, thereby reducing the radiation intensity. The absorption value is proportional to the sample’s thickness and the amount of absorbing substances. It is calculated from the inverse of the transmission: % refers to percent transmission, A* refers to the spectral absorption coefficient, and the terms Extinction (EXT), Concentration Unit (CU), and Absorption Unit (AU) are used.
Some of the light striking a sample is converted into other energy states within the material. In the case of an opaque sample, less light is reflected. For a transparent sample, this conversion of energy reduces the intensity of the transmitted (passing through) light. From the measured value of this transmitted energy, absorption can be calculated for each wavelength λ. Strictly speaking, since scattered light does not penetrate the sample, this is referred to as extinction (Eλ), where I0 is the energy of the incident light, and I1 is the measured energy of the transmitted light at that wavelength.
Here, I0 is the energy of the incident light, and I1 is the measured energy of the transmitted light at this wavelength.
I0 is the energy of the incident light and I1 is the measured energy of the transmitted light at this wavelength.